|
Many biological insights can be obtained by combining the power of Fluorescence Microscopy (FM) with that of Electron Microscopy (EM) to study the same sample – this is called Correlative Light and Electron Microscopy (CLEM). In FM, specific proteins can be labelled and identified, and their dynamics and interactions can be visualized in fixed or living cells. In EM, the full environmental context can be seen and high-resolution detail can be obtained. In this article we explain the concept of CLEM, with a particular focus on cryo CLEM: the combination of low-temperature FM with cryo EM. |
 |
When studying biological events within a cell or at the level of molecular interactions, scientists are often limited by the spatial resolution of FM. So-called "super-resolution" methods go some way towards overcoming this limitation to allow the localisation of individual fluorescent labels with a resolution reaching tens of nanometres. FM is also limited, because it visualises only the fluorescent marker and does not provide information on the structural and morphological context in which this marker is observed. This second limitation can also be an advantage as it provides specificity, allowing the labelled molecules to be identified in a dense sea of unlabelled molecules.
In contrast to observing specific labelled molecules, EM visualises local differences in electron density. This makes it an ideal technique to analyse the structure of macromolecular complexes or to describe cellular morphology and environment. In EM, however, it is difficult to directly distinguish specific types of molecules. In CLEM experiments, FM and EM are carried out on the same sample to link specific or dynamic information from FM, with high-resolution or environmental information from EM.
To understand the challenges of CLEM it is necessary to review the properties of an EM sample. In Transmission Electron Microscopy (TEM), an electron beam illuminates the sample and a projection image is recorded. When hitting the specimen, the accelerated electrons interact and are absorbed or scattered. The sample needs to be sufficiently thin for the electron beam to pass through the sample. Due to the vacuum present in the microscope column, samples also need to be imaged in a dry, solid state where they do not immediately disperse into the vacuum.
The most common preparation method of biological specimens for TEM is to sequentially replace all water in the sample by alcohols and then embed with a resin that is polymerised, resulting in a solid block. This block is then sectioned using a microtome to yield slices thin enough for TEM imaging. To enhance the signal and contrast of the EM image, electron-dense material such as heavy-metal stain can be added during sample preparation.
An alternative possibility for sample preparation is to rapidly freeze the sample to vitrify all the contained water. This can be achieved by quickly plunging grids containing thin specimens into liquefied gas, such as ethane or by using high pressure freezing devices with subsequent cryo sectioning. The benefit of this technique is that the sample remains hydrated in a close-to-native state and its molecular structure is optimally preserved. The resulting "cryo samples" can then be imaged in the EM (this is cryo EM), providing that they are kept at all times at temperatures close to that of liquid nitrogen.
Image contrast in cryo EM results only from the difference in electron density between the biomolecules and the surrounding ice. The images therefore include information on the molecular structure of the sample. Cryo EM can achieve resolutions of
Depending on the type of EM sample preparation being used, and depending on the type of FM being performed, one can use different CLEM modalities. One approach is to carry out live cell fluorescence imaging before preparing the sample for subsequent EM. This leaves a typical time gap of at least a few seconds between the states observed in FM and EM due to the time lost while fixing or immobilising the sample. Therefore this strategy is not ideal to study fast-moving processes or a very precise localisation of signals. A second approach is to carry out both EM and FM on the identical sample, once it has been prepared for EM. This procedure provides high correlation accuracy. This second approach can be applied to samples embedded in resin under particular conditions [1]. It can also be applied to cryo samples – this is cryo CLEM.
The particular challenges of cryo CLEM are that the specimen temperature must be maintained below –140 °C at all times to avoid formation of crystalline ice, and that the sample must not be exposed to humidity to avoid the condensation of water. These conditions must be maintained at all stages, including during FM, and while transferring the sample between the sample preparation device, fluorescence microscope, and the cryo EM equipped TEM.
Cryo CLEM requires a fluorescence microscope equipped with a special cryo stage that maintains low sample temperatures during imaging. The cryo stage must minimize contamination of the sample due to frosting from environmental humidity. The optical performance of an ideal system should enable high-resolution imaging with high sensitivity to allow high-accuracy correlation.
A number of cryo stages and associated workflows have been developed for cryo CLEM. These include both inverted configurations where the microscope objectives are separated from the sample by a glass slide as well as upright setups using long working-distance objectives. We have made use of an upright microscope configuration and a cooled objective front, allowing our cryo FM setup to employ a high NA objective with a short working distance while keeping the specimen vitrified for successful cryo CLEM.
Working with our original set-up, described in [2], provided experience that contributed to the development of the Leica EM cryo CLEM solution (Figure 1). This solution makes it possible to perform cryo CLEM in a more reliable and intuitive manner.

Fig. 1: The Leica EM cryo CLEM system.
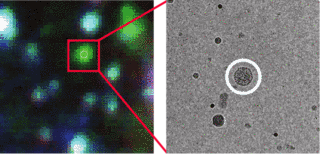
Cryo CLEM allows us to identify interesting biological objects (that could be dynamic, rare, or otherwise hard to find), based on their fluorescence signal. We can then locate the very same objects and obtain high-resolution structural data, in context, by cryo EM (Figure 2). In many cases such information cannot be obtained in any other way.
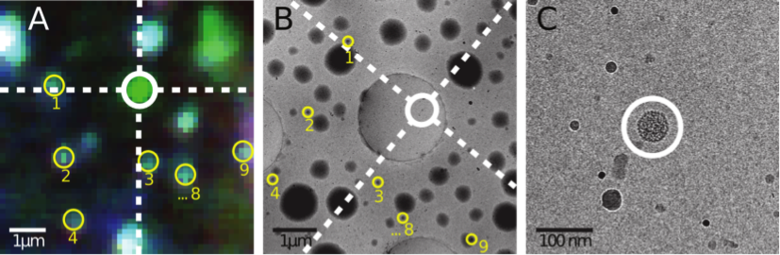
Fig. 2: A) Cryo FM image of a GFP labelled bacteriophage (circled in white). The multi-colour signals marked in yellow originate from fluorescent microspheres. B) Cryo EM overview image of the same part of the sample. The microspheres to marked in A are again marked in yellow. Their positions are used to locate the coordinates of the fluorescent bacteriophage in the cryo EM image. C) High magnification cryo EM image at the identified coordinates. The circle indicates the predicted origin of the signal, within which the bacteriophage is seen.