Kiến thức cơ bản về đo ORP
 |
ORP (mV) is short for oxidation-reduction potential. Nowadays, it is also called “redox potential.” In other words, it is the energy level (electric potential) determined by the equilibrium conditions between oxidants(Mz+) and reductants (M (z-n) +) that coexist in a solution. Suppose that only the following single equilibrium condition ① exists in a solution:
|

When a metal electrode (platinum, gold, etc.) and a reference electrode are inserted into this solution and the potential difference between these electrodes or ORP (mV) is measured by using a potentiometer (the millivoltmeter function of a pH meter), the potential difference can generally be expressed by the following equation:
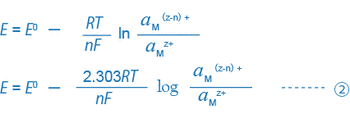
For example, equations ① and ② for a solution where trivalent and bivalent iron ions coexist can be expressed as follows:
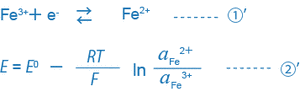
When only the single equation condition ①’ exists in the solution, the ORP of this solution is uniquely decided by equation ②’.
What is important here is that the ORP (mV) is decided by the ratio of the activity of the reductant (Fe2+) to that of the oxidant (Fe3+), or (aFe2+/aFe3+). Actually, many different equilibrium conditions between different ions exist in a solution at the same time. Thus, the ORP (mV) of such a solution cannot be expressed by this simple equation, and the physical or scientific meaning of the ORP found by the equation is not so clear.
Therefore, you need to understand that the ORP (mV) of a solution is one measure of the properties of that solution. On the other hand, the measurement of ORPs (mV) is widely used for analyzing solutions (potentiometric titration) or as a useful indicator in wastewater treatment.
Recently, it has been stated that a high ORP (mV) has a sterilizing effect and that if you drink water with a low ORP (mV), its reaction with active oxygen in the body’s cells makes you less susceptible to disease. Also, ORPs are used as an indicator for alkaline drinking water.
Types of Reference Electrodes and ORP (mV)
A measured ORP (mV) is the value for the reference electrode used. Thus, the ORP (mV) measurement of a solution appears to depend on the type of the reference electrode. HORIBA’s reference electrodes use Ag/AgCl with a 3.33 mol/L KCl internal solution.
In general academic papers, standard hydrogen electrodes (S.H.E. or N.H.E.) are often used as reference electrodes, and the relationship between the ORP (mv) when an N.H.E is used as the reference electrode and that when an Ag/AgCl electrode with 3.33 mol/L KCl is used as the reference electrode can be expressed by the following equation:

EN.H.E. :ORP (mV) measurement when a normal hydrogen electrode (N.H.E.) is used as the reference electrode
E :ORP (mV) measurement when a Ag/AgCl electrode with 3.33 mol/L KCl is used as the reference electrode
For more details, refer to “The Basis of pH - Electrical Potential of Reference Electrode.”
Positive and Negative Signs of Electric Potentials
In electrochemistry- or analytical chemistry-related books, examples like those shown below are used as standard oxidation-reduction potentials:
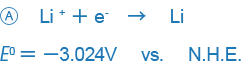
In some other books, however, the positive and negative signs are given in the opposite way (especially in books written in the U.S.).
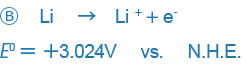
An example like B only expresses the reaction in the opposite way and there is no essential difference between the two expressions, but the difference in the way signs are used may be confusing. Currently, the way the sign is used in A is used in most places around the world, and we also this way of expressing ORPs.
This treaty is called the Stockholm Convention of the International Union of Pure and Applied Chemistry (IUPAC). Put simply, signs are used so that arrows indicate the direction in which the principal chemical species changes from an oxidant to a reductant.
Next page Measurement of ORPs 
|