Cryo-Transmission Electron Microscopy
An approach addressing both the problem of drying during specimen preparation and the issue of volatility of water in the microscope's vacuum is inspection of the samples in a special holder at –180 °C and below, in a frozen state with only minimal sublimation rate. This technique, known as cryo-transmission electron microscopy (cryo-TEM) or transmission cryo-electron microscopy has become an indispensable tool in structural biology over the last decades and, with the advent of cryo-electron tomography, also in cell biology.
For samples that are inherently thin enough such as isolated macromolecular complexes, viruses, or small cells, specimen preparation for cryo-TEM can be essentially reduced to one step: physical fixation by immersion freezing (plunge freezing). This article describes the basics and pitfalls of this technique, an accompanying article introduces several recent applications.
Immersion freezing

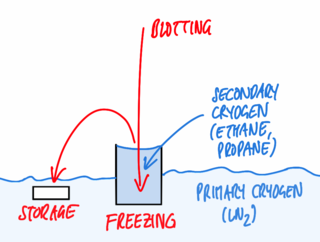
Fig. 1: Preparing the grid
One prerequisite for successful visualization of a sample embedded in ice is arresting the water molecules in place within a time so short not allowing the formation of ice crystals, ideally resulting in amorphously frozen (vitrified) water. In case of immersion freezing, this can be achieved only if a suitable cryogen with high thermal conductivity is being used and if the sample is immersed quickly to ensure quick freezing all over. Suitable cryogens include liquified ethane, propane, or a mixture of both [1] used just above the melting point (–183 resp. –188 °C for the pure compounds). Furthermore, the distance of any point in the specimen to the surface exposed to the cryogen has to short, i.e., the sample must be very thin.
Specimen carriers in cryo-TEM have to provide good support for the specimen, yet very little background not to reduce the low contrast of typically unstained cryo-samples any further. They also have to be conductive to avoid charging of the specimen and related problems such as drift. All these requirements are satisfied to some extent by standard TEM grids with a perforated carbon film, either of the lab-made type or the commercially available ones. The latter feature holes of very regular size and position easing automated data acquisition. Pre-treating the grids with glow discharging/plasma cleaning or coating with matrix proteins can be required to allow even spreading of aqueous solutions or attachment of cells.
For basic immersion freezing, a grid held by a forceps is fixed in a guillotine-like apparatus (Figure 1), a small quantity (3–5 µl) of the specimen in aqueous solution is applied, and the major part of the solution is blotted off with filter paper to reduce the thickness of the residual layer. This step is necessary both to allow vitrification (see above) and also to achieve a thickness that can be penetrated by the electron beam of the microscope, as for most samples, no further preparative steps are to be employed. The exact thickness depends on the sample and on the microscope available, typically ice layers as thin as the sample allows, but no thicker than 500 nm are being aimed for. This is also well within the range of thicknesses that can be frozen reliably by the technique described.
Immediately after blotting, the sample is plunged quickly into the cryogen. After freezing, the specimen can be transferred to LN2 to be stored almost indefinitely before inspection in the microscope.
Challenges

Fig. 2: The Leica EM GP semiautomatic immersion freezer.
A key issue is producing vitreous ice by sufficiently fast cooling, but also maintaining the ice in this amorphous state: the latter requires that the sample is kept constantly below recrystallisation temperature. This can be ensured by keeping the grid below LN2 or in a cold N2 gas atmosphere and pre-cooling all tools required for manipulation of the frozen specimen.
Another major issue is contamination of the frozen specimen by humidity condensing on its surface, forming nanometer size ice crystals. As the sample will be imaged frozen and as a whole (in contrast to methods like freeze substitution or Tokuyasu cryosectioning, where superficial crystals are removed by subsequent processing steps), this contamination will be evident as high contrast features in the TEM, that have to be avoided. This can be achieved by a number of provisions, aiming to reduce the humidity of the surrounding atmosphere and the time the sample is exposed to this atmosphere: typically, a dehumidified room is preferable, the sample should be kept below LN2, breathing onto the sample has to be avoided, all transfers should be performed quickly in the dry (and cold) N2 atmosphere just above the liquid phase using clean tools, and the microscope has to supply a clean vacuum in the objective area via a special anti-contamination device.
Even a perfectly frozen sample, however, will fail to produce results of significance if it was not treated well in the moments before freezing. One potential source of problems is the uncontrolled temperature of the specimen on an instrument as in Figure 1: located in an undefined position above a vessel containing LN2, the sample be exposed to room temperature or cold gases, leading to an ill characterized state.
On the same type of instrument, especially if working in a room with lowered humidity as recommended above, problems can arise from evaporation of the solvent: according to [2], water evaporates at a rate of 50 nm/s from a thin film at room temperature and a surrounding humidity of 30 %. This removal of solvent and the consequent enrichment of salts and other solutes can obviously critically influence the results obtained from aqueous specimens.
Even though basic instruments as the ones in Figure 1 have yielded very meaningful results in the hand of experienced researchers, many of the issues addressed above remained unsolved. Hence, labs have started building their own instruments with environmental chambers and automated blotting mechanisms. Commercial products by different manufacturers followed soon (for a review see [3]), including the Leica EM GP immersion freezer from Leica Microsystems, developed in collaboration with the IMP/IMBA Electron Microscopy Facility, Vienna (Figure 2).
References
- Tivol WF, Briegel A, and Jensen GJ: An improved cryogen for plunge freezing. Microsc. Microanal. 14: 375–79 (2008).
- Frederik PM, and Hubert DHW: Cryoelectron microscopy of liposomes. Methods Enzymol. 391: 431–48 (2005).
- Dobro MJ, Melanson L, Jensen GJ, and McDowall AW: Plunge freezing for electron cryomicroscopy in: Methods in enzymology 481: 63–82 (2010).